Research
Assembly of colloids in flow
Colloid, such as biopolymer or proteins, can be assembled for the fabrication of materials such as films, capsules, fibers, aggregates. Various shaping processes are employed, including 3D printing of liquid-liquid interfaces for creating structured liquids, emulsification for capsule production, and microfluidics for fiber formation. The applications of such materials span across diverse fields, including pharmaceuticals and the food industry. To achieve a rational design of these processes, understanding the relationships between colloid properties, assembly kinetics, and the physical (thermal, flow) and chemical conditions of the process is crucial.
My research focuses on controlling the structure and mechanical properties of micro-objects by manipulating physical and chemical process conditions. To achieve this, I employ techniques such as rheometry, microfluidics, microscopic imaging and scattering techniques.
We have focused on the self-assembly of biopolymers and charged surfactants at water/oil interfaces, demonstrating the nucleation and percolation kinetics of aggregate formation at the interface.
We have also developed membrane emulsification processes for high-speed production of microcapsules with controlled size and mechanical properties.
Another area of interest is the spinning of protein aggregates from milk and plant suspensions into fibers using microfluidics. We have demonstrated the spontaneous formation of core-shell materials through osmotic phenomena.
Currently, we are investigating the fabrication of core-shell materials using bio-sourced materials mechanically reinforced with cellulose.
Suspension of microcapsules with homogeneous size.
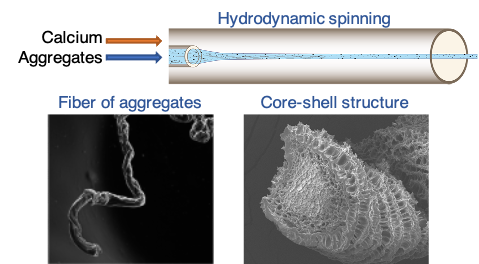
Hydorydnamic spinning of biopolymers for producing core-shell fibers
Controlling the spatio-temporal delivery of drugs or nutrients in the gastro-intestinal tract is of prime importance to improve pharmaceutical treatments or to control the nutritional properties of food products. The major constraints to such mixing are the high viscosity and the non-Newtonian characteristics of the gastro-intestinal content and the low velocity of the active mucosa, which together result in low Reynolds numbers. It is most likely that transfers in the lumen should limit biochemical reactions.
To address this, we have developed highly accurate quantification methods for intestinal motility and coupled them with realistic CFD models of intestinal fluid dynamics. Our investigations encompass both the scale of smooth muscle activity (1 mm) and the scale of villi, finger-like structures covering the intestinal mucosa with a length of approximately 500 μm. Remarkably, our findings demonstrate that the intestinal mucosa can be regarded as an active microfluidic mixer. Specifically, the movement of villi during longitudinal contractions serves as a significant radial mixing mechanism, enhancing dispersion and absorption near the mucosa despite the unfavorable rheological properties of the digesta.
We are undertaking a multidisciplinary approach that combines physiological experiments and computational fluid dynamics (CFD) modeling to develop a comprehensive and predictive understanding of microparticle transport in the gastrointestinal system. This research aims to enhance our knowledge of the complex dynamics involved in the movement of microparticles through the digestive tract, taking into account factors such as fluid flow, particle properties, and physiological conditions.

Observation of villi (stained in black) movements during mechanical activity of the small intestine.Lattice-Boltzmann simulation of mass transfers generated by this action.
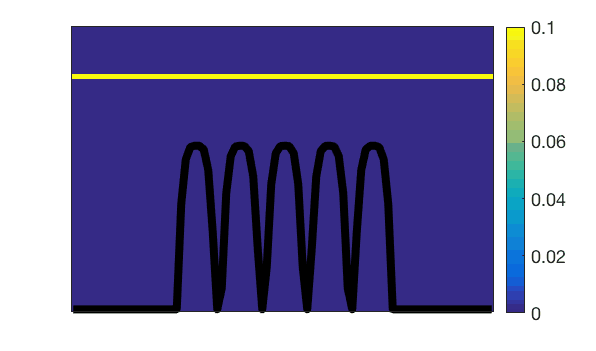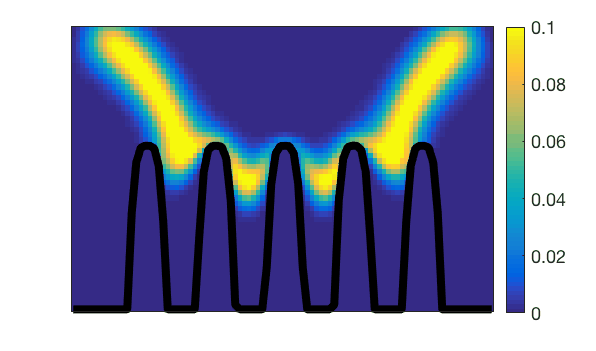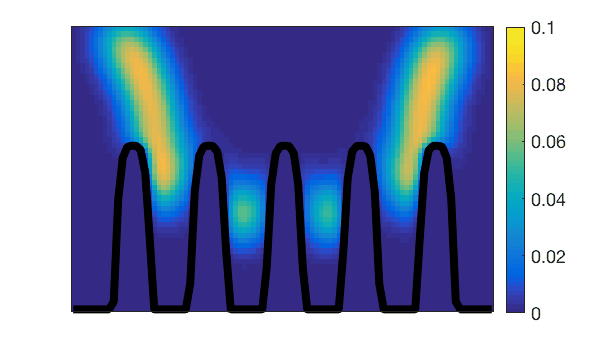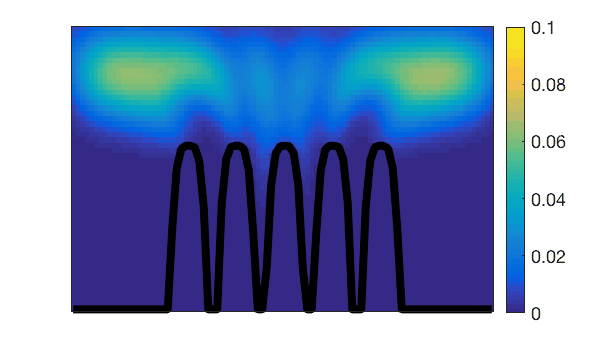 Lattice-Boltzmann simulation of mass transfers generated by the mechanical action of small intestine villi.
Lattice-Boltzmann simulation of mass transfers generated by the mechanical action of small intestine villi.
Microcapsules offer great potential as soft microparticles for precise spatio-temporal delivery of drugs, nutrients, and chemicals. Encapsulated within micro-drops and shielded by a thin elastic membrane, these substances are released through membrane rupture, fusion, or cyclic deformations. Notably, microcapsules exhibit high deformability under flow, making them biomimetic models of living cells. Our research focused on exploring the relationship between microcapsule deformation dynamics in simple flows and the rheological properties of their membranes.
Through the use of a unique cross-flow microfluidic setup, our experimental investigations have revealed the constitutive law governing membrane response through comparisons with numerical simulations and experimental data. In shear flow, we have observed cyclic and localized deformations in the microcapsule membranes. By quantifying and comparing these phenomena with numerical simulations, we have determined the membrane viscosity of these objects, which were previously assumed to be purely elastic. Furthermore, we have established the physical parameters and scaling laws that govern the instability of wrinkle formation in microcapsules under flow. Additionally, our research has demonstrated the occurrence of membrane restabilization in regimes of significant capsule deformations. Moreover, we have conducted a comprehensive study on the rupture of microcapsules, leveraging insights gained from the investigation of interfacial rheology and membrane structure.
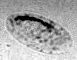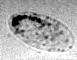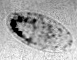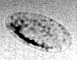 Tank-treading of a microcapsule in shear flow.
Tank-treading of a microcapsule in shear flow.
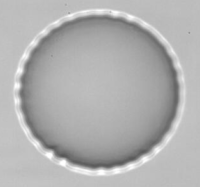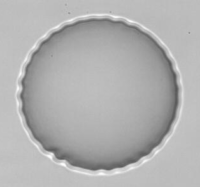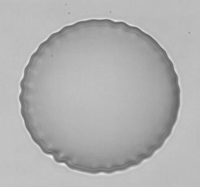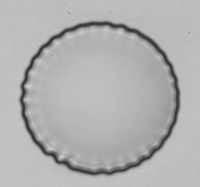 Wrinkling instability observed with an original flow chamber, PhD Thesis of Kaili Xie.
Wrinkling instability observed with an original flow chamber, PhD Thesis of Kaili Xie.
Food oral processing helps us to understand how food properties, the physiology of the mouth, and sensory perception interact to influence food enjoyment. This knowledge can lead to the development of healthier and tastier food products, personalized nutrition recommendations, and interventions to improve the swallowing function of individuals with dysphagia or other swallowing disorders. During my PhD thesis, I focused on the importance of understanding and modeling the phenomena that govern stimuli release during food consumption.
One of my main result was the development of a model for salt release during mastication of solid foods. The breakdown was comprehended by the generation of the area of contact between the product and the saliva that governs the mass transfers of salt. Fitted on in-vivo data, this model showed that salt release was correlated to the masticatory performance of the individual as well as the breakdown behavior of the product, which could be determined through rheological characterization.
In addition, I also investigated the biomechanics of swallowing during the pharyngeal stage, with a focus on the coating of the pharyngeal mucosa and the release of aroma compounds in this layer. I developped an elastohydrodynamic model of pharyngeal mucosa coating and showed that the food bolus coating the mucosa is very diluted by saliva during the swallowing process. Consequently, the impact of product viscosity on flavour release is weak.
